After finding the SEPT product you want to
purchase, click on BUY and the system will transfer
you to the NORMADOC store which provides the fulfillment process for SEPT.
Every checklist is available in PDF or word format. The latter format allows you to adapt the checklist to your business case and/or a media of your choice such as a web-based excel worksheet. This enables you to demonstrate compliance with the standard in a manner that is most efficient for you.
Every checklist comes with four hours of free consultation. SEPT will answer any question concerning the standard or checklist for 60 days after purchase.
Checklist for - IEC 62304:2015 Medical Device Software - Software Life Cycle Processes
Author: Stan Magee Pages: 130
IEC released amendment 1 for IEC 62304 in June of 2015. The purpose of this revision was:
- Additional requirements to address software life cycle processes specific to legacy software
- Clarification of requirements and updates for Software Safety Classification to include a risk-based approach, focus on overall medical device risk analysis. With a strong reference for using ISO 14971 processes
- Minor revisions to over 40% of the standard.
- The standard has over 160 required and over 160 suggested policy, procedure, plan, record, document, audit, or review
This checklist addresses the amendment and the base standard .
IEC 62304:2015, is often confusing and laborious. This is because directions contained in the standard can seem unclear or ambiguous. To aid in determining what is actually required by IEC 62304, the experts at SEPT have produced a checklist. This checklist was prepared by analyzing each clause in IEC 62304 for key words that signify a required policy, procedure, plan, record, document, audit, or review.
This revised standards has over 160 required artifacts and over180 suggested ones. Below is a table showing the required artifacts by type.
Required Artifacts
| Procedures | Plans | Records | Documents | Audits | Reviews | Total |
| 25 | 12 | 69 | 46 | 1 | 10 | 163 |
The resulting checklist then provides an easy-to-use categorized list of physical evidence against which you can audit your work products to help insure conformance with IEC 62304.
To better understand this model a user of this product should understand SEPT definition of an auditor. An auditor can be your boss, an inside auditor or an outside auditor such as the European Union, FDA or a prime contractor for your product. The checklist will give the auditor and you a common reference point in the standard (Clause number) that becomes the index point for physical evidence. If a standard calls out physical evidence more than once , such as a "training plan" it is always index to the first clause that reference the training plan This method will allow you to organize you physical evidence in a systematic manner for presentation to the auditor. This simple checklist allows you to bring the document down to simple terms that a professional lay person can understand (policy, procedure, plans, records, documents, audits and reviews).
The checklist will allow the organization to divide the compliance activity into manageable work packages such as procedures, plans, documents etc.
The checklist is available in PDF or word format. The latter
format allows you to tailor the document to your business case or
the media that your organization wants to use the checklist in
such as excel web page format or any other end product type in
order to meet compliance with the standard in the most efficient
way possible.
Checklist for - ISO 13485:2016 Medical Devices - Quality Management Systems - Requirements for Regulatory Purposes
Author: Andy Coster Pages: 176
This is a checklist for ANSI/AAMI/ISO Standard 13485:2016.
This standard is a requirement for all medical devise producers.
It goes much further than ISO 9001 in requirements for
documentation; and represents a major change in concept,
being a "stand-alone" quality system standard for
medical devices. This checklist clarifies what is required
for compliance by providing an easy-to-use product evidence list that
will assist any organization to meet the requirements of this important
standard.
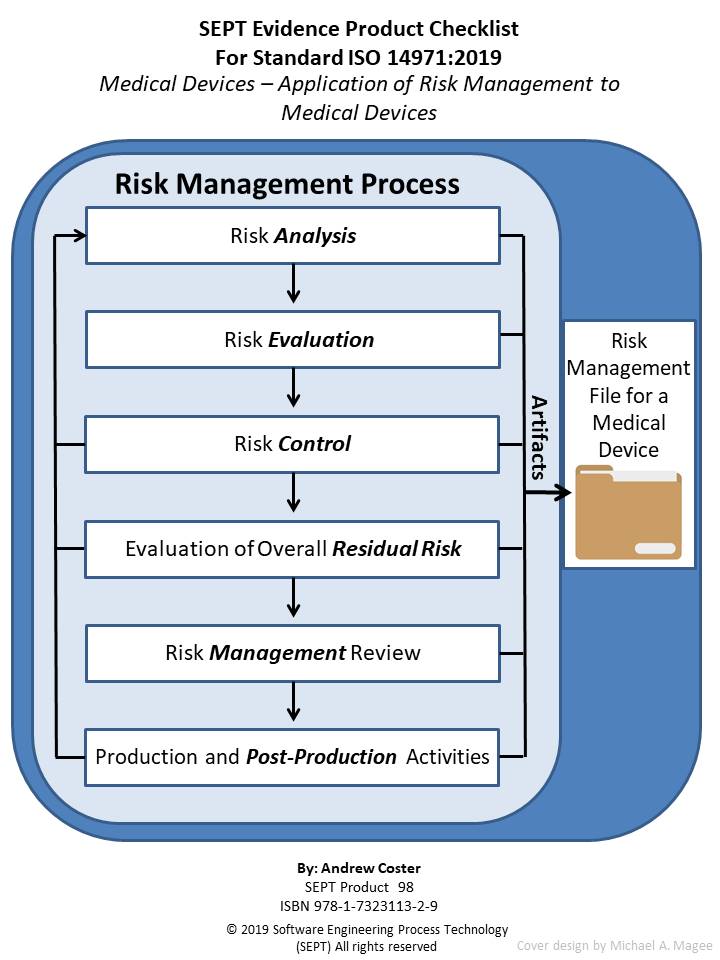
Checklist for - ISO 14971:2019 - Application of Risk Management to Medical Devices
Authors: Andy Coster Pages: 143
For 25 + years Software Engineering Process Technology (SEPT) has produced checklists for international standards. They have been produced for Medical Devices, Quality, Security and Software processes. Organizations buy and use these checklists to:
- Perform a gap analysis to show what the Standard requires requires versus what the organization does;
- Ensure that all artifacts cited in the standard are addressed;
- Provide traceability from each artifact in the standard to an organization's process step;
- Demonstrate that the organization has followed an international standard in case of litigation;
- Provide an economical, off-the-shelf checklist rather than a costly, internally-developed checklist; and
- Insure the use of a checklist that has been compiled by a company experienced in deciphering international standards and has been verified by domain experts.
This is a checklist for ISO 14971:2019, another checklist related to medical device standards. The purpose of the checklist is to define clearly all the artifacts (policy, procedure, plan, records, document, or reviews) that the underlying standard calls out. Normally the SEPT checklist has a section for the artifact "audits". However, the ISO 14971:2019 standard does not specify any requirements for "audits" so that section is left blank for this checklist. Nevertheless, in many sections there is a requirement to inspect the risk management file. Furthermore, what constitutes physical evidence (Artifacts) to meet the guidance outlined in ISO 14971 is sometimes difficult to identify. To bridge this gap the author and SEPT experts have identified items of physical evidence called out in the standard based on their knowledge of the document and their experience in the standards field. Each item of physical evidence that was identified by these experts is listed in the checklist as an artifact (policy, procedure, plan, records, document, or reviews.)
There must be an accompanying record of some type when a review has been accomplished. This record would define the findings of the review and any corrective action to be taken. For the sake of brevity this checklist does not call out a separate record for each review. All procedures should be reviewed but the checklist does not call out a review for each procedure, unless the standard calls out the procedure to be reviewed.
The author has carefully reviewed the Standard ISO 14971:2019 and defined the physical evidence required based upon this classification scheme. SEPT's engineering department has conducted a second review of the complete list and baseline standard to ensure that the document's producers did not leave out a physical piece of evidence that a "reasonable person" would expect to find. If an artifact is called out more than one time, only the first reference is stipulated. If an artifact is required by ISO 14971:2019, it appears in the checklist without an appended symbol. If an item is "suggested" either by ISO 14971:2019 or by inference when a document or plan is called out it appears with an appended asterisk (*). If an item is required to be included in the associated Risk Management File by ISO 14971:2019 it appears in the checklist with an appended greater-than symbol (>). In this way traceability of requirements and suggested items as well as the need to include a required item in the Risk Management File is possible.
The checklist is available in PDF or word format. The latter format allows you to tailor the document to your business case or the media that your organization wants to use the checklist in such as excel web page format or any other end product type in order to meet compliance with the standard in the most efficient way possible.
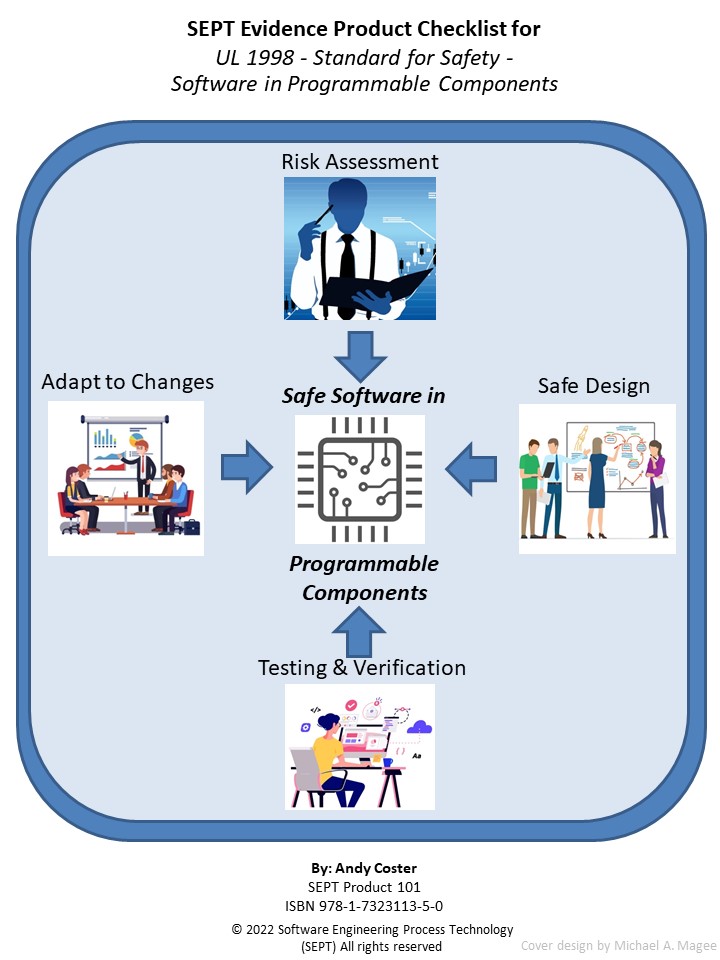
Evidence Product Checklist for UL 1998 Standard for Safety -Software in Programmable Components, Edition 3
Authors: Andy Coster Pages: 97
Overview
If a company produces products that have software in a programmable component that possibly could cause bodily harm, they need to adhere to safety standard UL 1998. This SEPT checklist is designed to ensure that the required artifacts (procedures, plans, records, documents and reviews) have been produced to meet the requirements of UL 1998. While helping you meet the UL standard, it will also save you time and money while doing so.
The UL 1998 Standard
The UL 1998 standard is focused on application-specific, non-networked software in a programmable component that is embedded in a product for which a failure may result in injury to persons. In addition, UL 1998 is a reference software standard intended to be used in conjunction with any product specific safety standards that address safety requirements for the identified programmable component and the product hardware.
The software configuration of a microprocessor based programmable component typically includes the operating system or executive software, communication software, micro-controller, input/output hardware, and any generic software libraries, database management or user interface software.
Background of SEPT Checklists
For 25 + years Software Engineering Process Technology (SEPT) has produced checklists for international and national process standards. They have been produced for Medical Devices, Quality, Security, and Software processes.
Organizations buy and use these checklists to perform a gap analysis between what a particular standard requires and what their organization does. This
- Ensures that all the artifacts cited in the standard are addressed,
- Provides traceability between artifacts in the standard and the organization's process step that produces the artifact,
- Demonstrates that the organization has followed “best practices” in case of litigation,
- Reduces cost by procuring an “Off the Shelf” checklist rather than developing one internally,
- Ensures use of a checklist compiled by a company experienced in deciphering standards and verified by domain experts.
How the UL 1998 SEPT Checklist was Constructed
The first step in construction of the checklist was to determine all of the artifacts (procedures, plans, records, documents, or reviews) that the standard requires. Sometimes what constitutes required physical evidence (artifacts) to meet the UL1998 standard is difficult to identify. To bridge this gap the author and SEPT experts have identified items of physical evidence called out in the standard based on their knowledge of the document and their experience in the standards field. Each item of physical evidence that was identified by these experts is listed in the checklist as an artifact - a procedure, a plan, a record, a document, or a review.
Sometimes we call out an artifact that is not expressly required by the standard. In such cases, we flag that artifact with an asterisk (*) to indicate we suggest that it be produced even though it is not specifically called out by the standard. If an artifact is specifically called out by UL1998, it appears in the checklist without an appended symbol.
Whenever a review takes place, there must be an accompanying record of some type indicating that the review has been performed. This record would define the findings of the review and any corrective action to be taken. For the sake of brevity this checklist does not call out a separate record for each review. All procedures should be reviewed but the checklist does not call out a review for each procedure unless the standard calls out that the procedure be reviewed. Where records are cited as artifacts, reviews of the records are not included, but we would expect a review to be held.
The author has carefully reviewed the UL1998 Standard and defined the physical evidence required based upon the classification scheme of procedures, plans, records, documents, and reviews. SEPT’s engineering department has conducted a second review of the complete list and baseline standard to ensure that the documents’ producers did not leave out a physical piece of evidence that a “reasonable person” would expect to find. If an artifact is called out more than one time, only the first reference is stipulated.
There are occasional situations in which a procedure or document is not necessarily separate and could be contained within another document. For example, the “Software Design Operation and Safety Features Document” could be a part of the “Software Design Document”. The author has called out these individual items separately to ensure that the organization does not overlook any facet of physical evidence. If the organization does not require a separate document, and an item can be a subset of another document or record, then this fact should be denoted in the detail section of the checklist for that item. This should be done in the form of a statement reflecting that the information for this document may be found in section XX of Document XYZ.
If organizational requirements do not call for a physical evidence for a project, this should also be denoted with a statement reflecting that the physical evidence is not required and why. The reasons for the evidence not being required should be clearly presented in this statement. Further details on this step are provided in the Detail Steps section of the introduction. The size of documents called out in the checklist and the standard itself can vary from paragraphs to volumes depending upon the size and complexity of a project or business requirement.
Required artifacts gleaned from the UL1998 standard have been transferred into checklist tables, based on the type of artifact and the standard section in which they occur. In total, there are 208 artifacts identified by SEPT in the checklist, of which 69 are required and 139 suggested.
Checklist for - FDA, Electronic Records; Electronic Signatures; Final Rule-FDA 21CFR Part 11
Author: Stan Magee Pages: 31
This is a checklist to help you meet FDA requirements for
electronic records and signatures. The checklist
uses a classification scheme of physical evidence comprised of
procedures, plans, records, documents, audits, and reviews. Identifying
over 50 pieces of physical evidence, this checklist
clarifies what is required for compliance to this standard by
providing an easy-to-use product evidence list.
A quality product at a reasonable price, this checklist will save you
time and money, and aid you in
meeting certain governmental requirements.
New! Interested in an
unlimited 5-year corporate license for this product?
Contact Stan Magee at stanmagee@smartwire.net.
Checklist for FDA, General Principles of Software Validation Final Guidance for Industry and FDA staff as amended by “Guidance for Industry, FDA Reviewers and compliance on Cyber security for Networked Medical Devices Containing Off-the Shelf (OTS) Software"
Author: Stan Magee Pages: 110
Now! The experts at SEPT have produced a checklist for this major software engineering standard: "General Principles of Software Validation - Final Guidance for Industry and FDA staff. The Checklist uses a classification scheme of physical evidence comprised of procedures, plans, records, documents, audits, and reviews. The Checklist clarifies what is required for compliance by providing an easy-to-use product evidence list that will assist any software organization in meeting the requirements of this guide. A quality product at a reasonable price; this checklist will save you time and money, in meeting governmental requirements.
Checklist for - FDA, Guidance for Industry, FDA Reviewers and Compliance on Off-the-Shelf Software Use in Medical Devices as amended by Guidance for Industry, FDA Reviewers and Compliance on Cyber security for Networked Medical Devices Containing Off-the Shelf (OTS) Software, January 14, 2005
Author: Stan Magee Pages: 22
The experts at SEPT have have created a checklist to help ensure that the
you have complied with the requirements specifed in the FDA document,
"Guidance for Industry, FDA
Reviewers and Compliance on Off-the-Shelf Software Use in
Medical Devices, as amended in 2005 by FDA's guidance on compliance for
cybersecurity.
The Checklist
provides an easy-to-use classification scheme of physical
evidence comprised of procedures, plans, records, documents,
audits, and reviews. The Checklist clarifies what is required
for compliance through a product evidence list that will assist
any software organization in meeting the requirements of these FDA
guideline.
Use of our checklist will save you time and money, and may aid in
meeting governmental requirements.
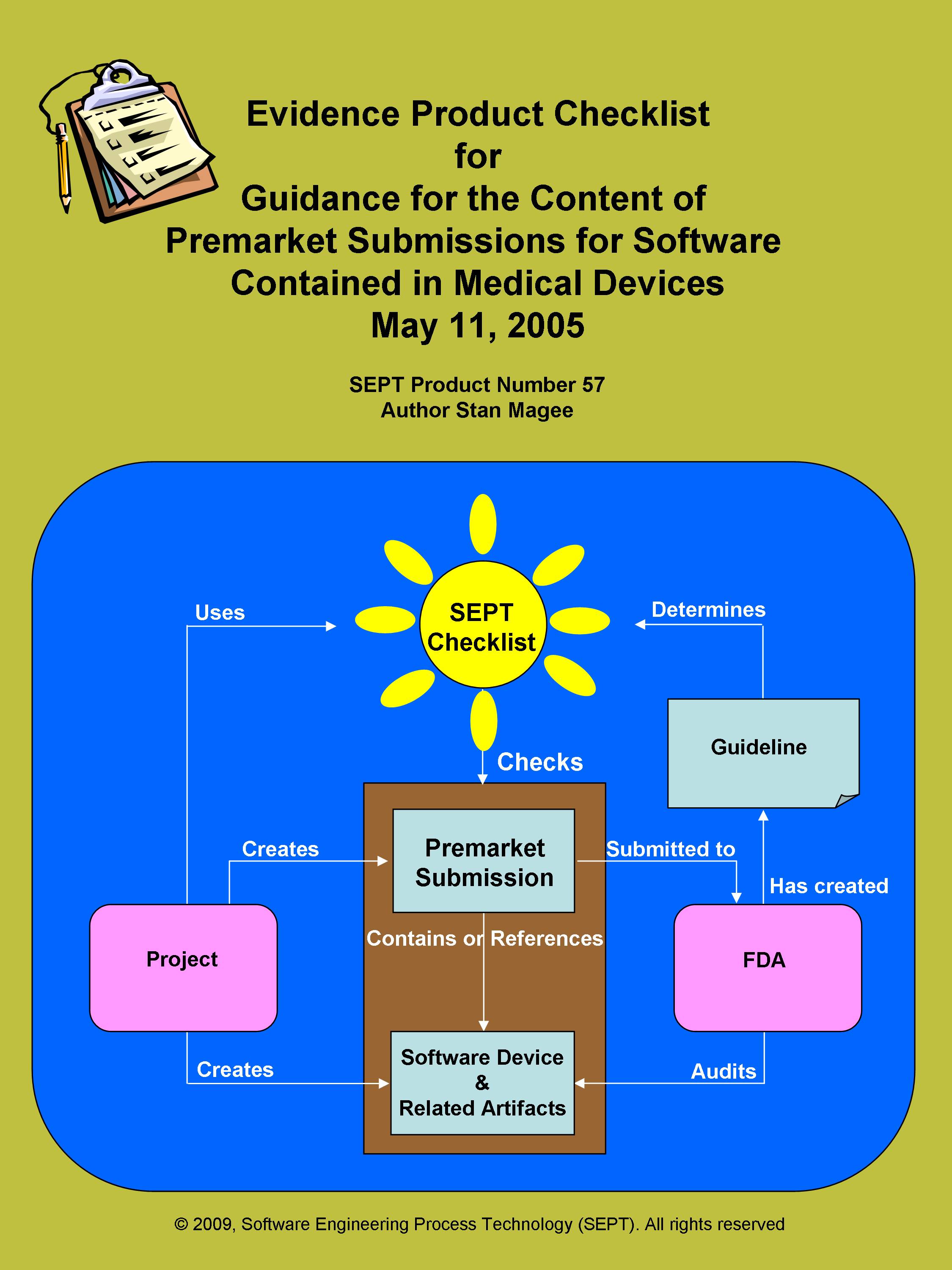
Checklist for - FDA, Guidance for the Content of Pre-market Submissions for Software Contained in Medical Devices.
Author: Stan Magee Pages: 83
SEPT has produced a checklist for the FDA Document, "Guidance for the Content of Pre-market Submissions for Software Contained in Medical Devices." This is a "must have" for all quality managers and engineers involved in this FDA document. The Checklist uses a classification scheme of physical evidence comprised of procedures, plans, records, documents, audits, and reviews. This standard calls out or suggests over 120+ items of physical evidence. The Checklist clarifies what is required for compliance by providing an easy-to-use product evidence list that will assist any organization to meet the requirements of this important standard.
New! Interested in an unlimited 5-year corporate license for this product? Contact Software Engineering Process Technology (SEPT) for more information!